Abstract
Background: Venetoclax has shown promising activity when combined with hypomethylating agents (HMAs) and low-dose cytarabine (LDAC) with response rates in the range of 75% in untreated patients ≥65 years with acute myeloid leukemia (AML). [DiNardo C, ASH 2015 Abstract 327 & Wei A, ASH 2016 Abstract 102]. Given the favorable data in the first-line setting, we aimed to assess the efficacy of combining venetoclax with either HMA or LDAC in patients with relapsed or refractory (R/R) myeloid malignancies.
Methods: This is a retrospective, single-center review of all patients with a myeloid malignancy treated with venetoclax plus either azacitidine (AZA), decitabine (DEC), or LDAC. Patients treated between May 1, 2016 and June 7, 2017 were included in this analysis. After a 5-day ramp up period, a target dose of venetoclax 600 mg PO daily was used when combined with LDAC. Venetoclax was given continuously starting on cycle 1 day 1 and continued until progression or toxicity. Cytarabine was given 20 mg/m2 SC daily on days 1 - 10. Subsequent cycles were started at discretion of the treating physician, but typically began on day 29 to 36. When venetoclax was combined with HMA, the target venetoclax dose was 400 - 800 mg, after a 5-day ramp up period during cycle 1. AZA and DEC dosing regimens and schedules were consistent with standard of care. The dose of venetoclax was reduced by 75% and 50% in patients receiving a strong CYP3A4 inhibitors (i.e. voriconazole or posaconazole) and moderate CYP3A4 inhibitors (i.e. isavuconazole), respectively. Responses were graded using European Leukemia Net (ELN) response criteria. Overall response rate (ORR) was defined as rate of complete response (CR) + complete response with incomplete hematologic recovery (CRi) + partial remission (PR). Hematologic improvement (HI) was classified as continued improvement until next cycle of treatment in erythroid, platelet, or neutrophil response per IWG Criteria for myelodysplastic syndrome [Cheson BD, Blood. 2006]. Immunophenotypic minimal residual disease (MRD) was identified in bone marrow aspirates by multiparameter flow cytometry. Next generation sequencing was performed on bone marrow samples to identify characteristics of responders and non-responders.
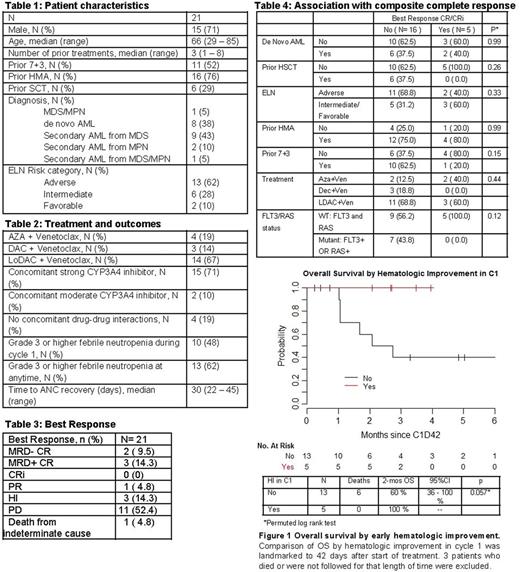
Results: As of June 7, 2017, 24 patients have been treated with the combination of venetoclax and HMA (n=8) or venetoclax and LDAC (n=16). 21 patients were evaluable for response to cycle 1. (Tables 1 and 2) 6 out of 21 patients achieved PR or better on venetoclax regimens with ORR of 28.6% (95% CI 11.3-52.2%). (Table 3) 5 out of 21 patients (23.8%) experienced CR. No CRs were seen in 6 patients treated after prior allogeneic stem cell transplant (alloSCT). (Table 4)HI was observed in 8 out of 21 patients (38.1%). Patients who showed HI were more likely to have had no prior 7+3 compared to patients who did not show HI (87.5% vs. 23.1%, p=0.008). Other factors were not associated with HI. Median follow-up among survivors from start of treatment was 4.1 months (range 1.2- 13.4). 8 out of 21 patients died, and 3-month OS was 72% (95%CI 54-97%). Based on landmark analysis, 5 patients achieved HI by 42 days following treatment start. 2-month OS for patients who achieved HI by 42 days was 100%, compared with 60% among 13 patients who did not achieve HI by 42 days (p=0.057). (Figure 1) No CRs were seen in patients with FLT3 or RAS mutations, but a larger cohort may be necessary to further evaluate molecular predictors. There was no significant association between ELN Risk Group and response. Notably, CRs were seen even among ELN adverse risk patients, including one 50 year-old man with AML associated with inv(3), refractory to induction chemotherapy, high-dose cytarabine, investigational BET inhibitor, and salvage chemotherapy with mitoxantrone, etoposide, and cytarabine, who achieved MRD+ CR after 1 cycle of AZA + venetoclax and went on to alloSCT. One other patient with inv(3) did not respond after 2 cycles of LDAC + venetoclax.
Conclusions: Venetoclax in combination with HMA or LDAC shows activity in patients with R/R myeloid malignancies. Although data are limited by small numbers and short-term follow-up, there is a trend toward prolonged OS for patients who achieve early HI within 42 days of treatment initiation. Updated responses and survival estimates for all patients will be presented, including those evaluable after data cut-off.
Goldberg: ADC Therapeutics: Research Funding; Pfizer: Research Funding; Genentech: Research Funding. Horvat: Agios: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Mauro: Bristol-Myers Squibb: Consultancy. Stein: Constellation Pharma: Research Funding; Pfizer: Consultancy, Other: Travel expenses; Seattle Genetics: Research Funding; GSK: Other: Advisory Board, Research Funding; Novartis: Consultancy, Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Research Funding; Celgene Corporation: Consultancy, Other: Travel expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.